Describe the Movement of the Particles in the Air
PM stands for particulate matter also called particle pollution. Theoretically then they cancel each other out in the vector sum.
How Are Particles Arranged In The Three States Of Matter Quora
-Imagine a box full of air particlesThe particles are forced to move to a point A on the edge of the boxMy question is nowhow can I mathematicly describe the movement of these particles toward point A using one generalised equation.
. Warm air is lighter and it rises upwards meanwhile cold air is denser and hence it moves down to replace the warm air. Describe how different energy stores are changed by the boiler. 102 understand the interconversions between the three states of matter in terms of.
The oscillation of one set of particles has a huge impact on neighboring particles c particles become too close together as they bump into. Air flows horizontally at top of the troposphere. The particles collide with each other and with the walls of any container in which they are held.
They move with a range of different speeds. What happens to the movement of the air particles. It could be dangerous if the temperature of the air inside the canister increased by a large amount.
2 marks 2 and 4. They vibrate about a fixed position. Up to 24 cash back As it travels the air makes rapid swirls of movement in order to cause small particles in the air to stick to mucus.
2 marks Tick TWO boxes. The pharynx is divided into three. As part of the grants educational piece Ferro developed an educational unit for K-12 on indoor air quality and re-suspension of particles taught in local schools by a.
Freezing movement of particles. Newtons third law says that all these internal forces act in pairs action and reaction. The motion of all the particles is predictable.
Mary put out the fire. They move in random directions. Observe the motion of the three colored air particles.
Warm air rising creates a low pressure zone at the ground. This operation is called particle movement prt movt. The particles in a gas are moving very quickly in random directions.
3 marks 0 5 4 To heat the house the boiler transfers 15 MJ of energy in 10 minutes. Some evidence for the movement of particles in gases is when the perfume gas molecules diffuse into the air when you put it on because the particles from the perfum diffuses with the gas particles around you and it spreads all over your room. The speeds of the particles vary but on average they move quicker than they do in.
The pharynx is a pathway in which both air and food travel henceforth it is an important passage for the digestive and respiratory tracts. 1 Which TWO sentences describe the movement of the air particles in the canister. Horizontal flow is called advection.
The volume of the balloon increases as the. See answer 1 Best Answer. The movement that gas particles make is they vibrate very fastand rapidly they are also all spread far.
In liquids there is a mild force of attraction between the particles it holds them together but at the same time allow their free movmentwhile in a gasThere is no force of attraction between the particlesso they move randomly Answer link. Turn over 20 20 BLANK PAGE 21 21. How does the motion of the particles change when the changes from a solid to a liquid to a gas.
These particles are in constant random motion 3. The term for a mixture of solid particles and liquid droplets found in the air. Do not write outside the box.
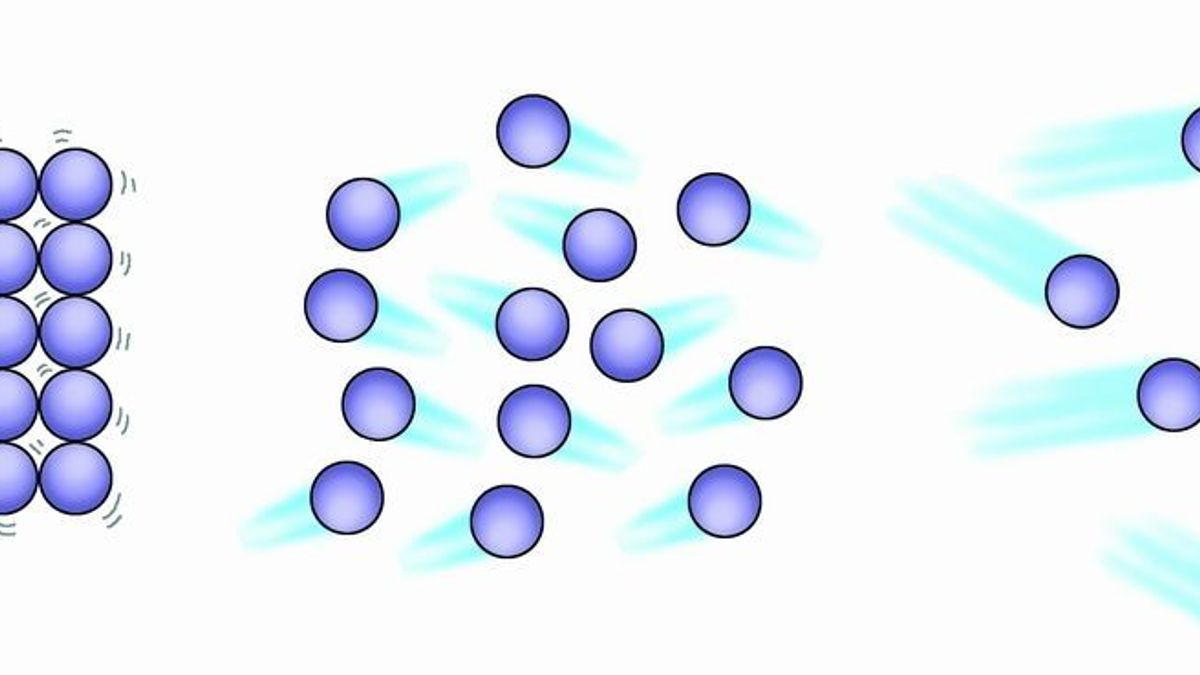
Which two sentences describe the movement of the air particles in the canister. Up to 24 cash back Movement of Particles - IGCSE Chemistry MrRichard by Save and Karan 10S. 101 understand the three states of matter in terms of the arrangement movement and energy of the particles.
The amount of energy that the particles lose from these collisions is negligible. Figure 1 a Which two sentences describe the movement of the air particles in the canister. A few basic principles go a long way toward explaining how and why air moves.
In this case the transformation is obligatory. Vibration in fixed positions. Particles movement is determined by the energy they have and their relation to other particles.
See picture Thank you for your time. Correct answer - Describe the movement of air over 2 nearby land areas one of which is heated more than the other. The air cools until it descends.
It is also humidified filtered and warmed. The movement of air is mainly caused by the differences in pressure and temperature. Water changing to a solid.
They move in random directions. The motion of all the particles is predictable. Air from the surrounding area is sucked into the space left by the rising air.
The particle movement transformation is optional in subject-verb-direct object sentences with particles except when the direct object the NP is a personal pronoun. Others are so small they can only be detected using an electron microscope. 1 mark 8 08 IBMJun188464P1H.
Mary put the fire out. The temperature of the air inside the canister increases. Some particles such as dust dirt soot or smoke are large or dark enough to be seen with the naked eye.
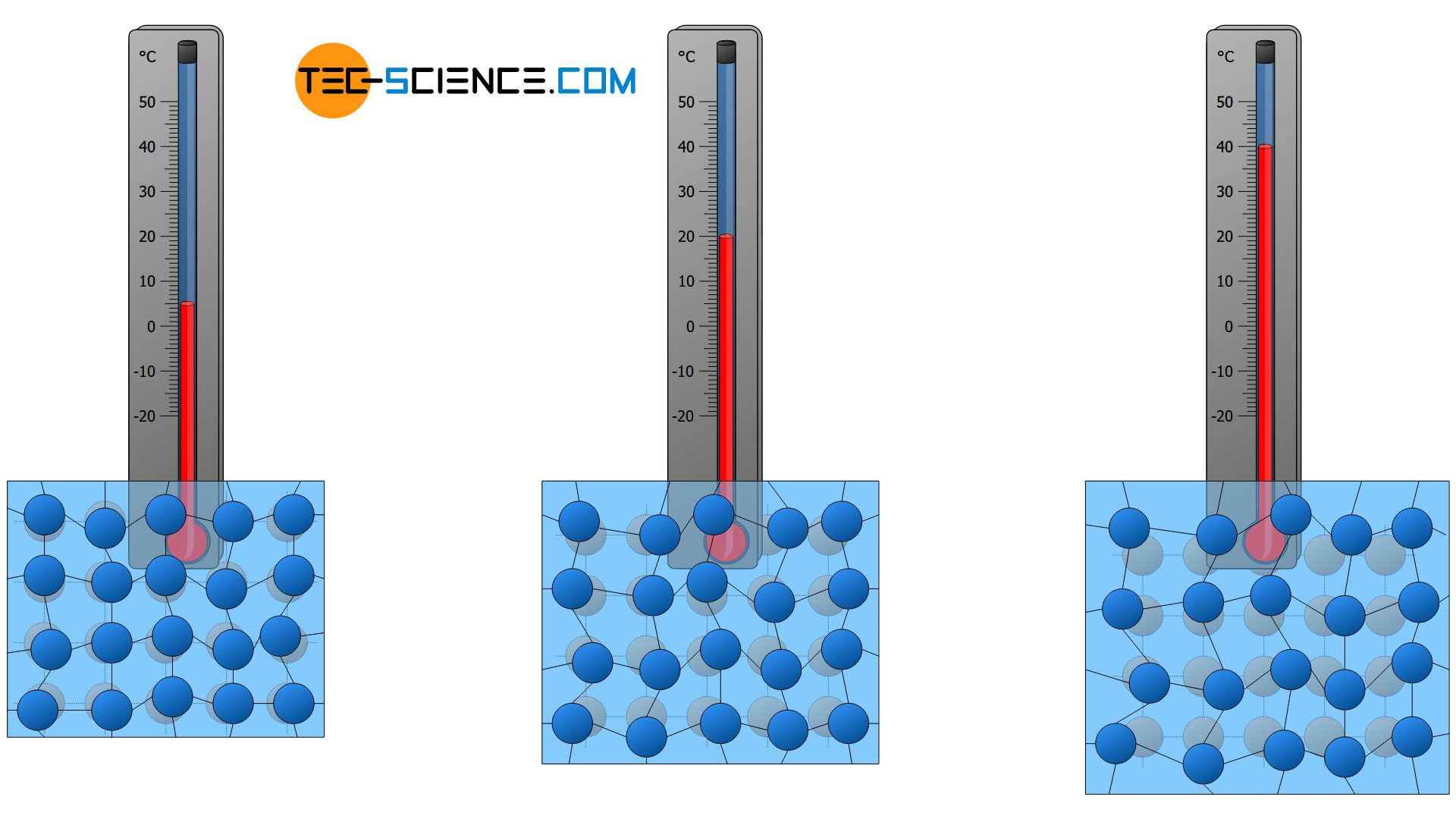
Describe the motion of these particles for a sound wave moving through air. The names of the interconversions how they are achieved and the changes in arrangement movement and energy of the particles. This allows the particles to overcome the forces of attraction in a solid which means the regular pattern is broken down and the particles can slide over each other.
Answers and Replies Jul 7 2015 2 mfb. Up to 24 cash back What happens to the movement of the air particles. They reach a similar point and come together as the wave reaches the highest point.
They move with a range. The particles in a gas are moving very quickly in random directions. All matter is composed of tiny particles atoms molecules and ions 2.
They reach a similar point and come together as the wave reaches the highest point and then drift separate at the lowest point. Among all the forces acting on the particles will be the internal forces that the particles exert on one another. Which makes you able to SMELL it.
They vibrate about a fixed position. Gas particles move in a straight-line motion If the temperature of the air in the balloon increases the molecules move faster and have more collisions. The particles lose kinetic energy to a point where the forces of attraction are able to hold the particles together tightly.
They move in circular paths. The particles gain kinetic energy to a point where they vibrate faster. The speeds of the particles vary but on average they move quicker than they do in.
This phenomenon creates wind.
Temperature And Particle Motion Tec Science

Comments
Post a Comment